中国团队首次提出“纳米催化医学”新概念
来源:《化学学会评论》
作者:施剑林等
时间:2018-04-25
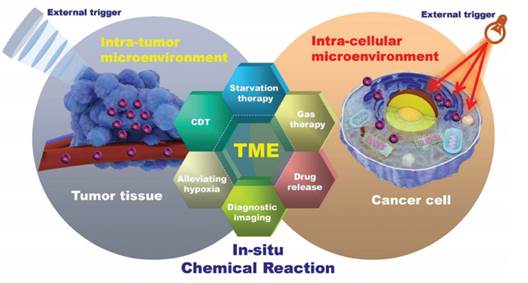
近期,中国科学院上海硅酸盐研究所施剑林研究员和陈雨研究员带领的研究团队在国际权威综述学术期刊Chemical Society Reviews(影响因子:38.618)发表综述论文“Nanoparticle-Triggered In-Situ Catalytic Chemical Reactions for Tumour-Specific Therapy” ,首次提出“纳米催化医学”的新概念(Nanocatalytic Medicine, NCM)。
研究团队对这一概念进行了精确的定义和全面的阐述,并将这一新的方法应用在肿瘤的高效、安全治疗中,为纳米医学和临床医学的发展开辟了一个全新的研究领域。
常规的肿瘤化疗采用具有高细胞毒性的化疗药物,会同时杀死癌细胞和破坏正常细胞通过细胞凋亡或非选择性坏死的途径。如果能够采用无毒/低毒的纳米颗粒,通过选择性地催化/触发肿瘤组织内部的特定化学反应,在局部产生数量可观的特定反应产物,则可以实现一系列的生物学和病理学响应,这可能在不对正常组织产生显著副作用的情况下,实现肿瘤特异性的治疗和成像,以达到特异性的癌症防治目的。
然而,利用催化化学反应实现选择性肿瘤诊疗的相关技术进步,依赖于基础化学、材料科学、纳米技术和生物医学等多学科领域的交叉融合与技术革新,这个新兴的跨学科研究有别于传统的临床癌症诊断模式。为了总结基于肿瘤局部催化化学反应的癌症研究发展动态,该综述汇集了以巧妙设计的化学组成、结构、理化和生物学效应的纳米平台为基础的,具有多功能性、选择性催化反应响应的癌症治疗模式,这些催化反应的响应源包括:内源性的肿瘤微环境(TME)和外源性的物理场刺激。
这些独特的肿瘤内化学反应可用于肿瘤窒息饥饿疗法(TME响应的催化反应剥离瘤内氧组分与养分)、化学动力学疗法(TME响应的催化反应原位产生高毒性活性氧组分)、声动力学治疗(超声刺激瘤内原位化学反应产生有毒组分)、气体治疗(TME响应或外场催化反应产生治疗性气体),逆转肿瘤乏氧(TME响应或外场催化反应提高氧含量),以及肿瘤微环境响应的诊断成像和对多种刺激响应的药物释放,甚至其他外场催化的多样性诊断与治疗。
为了更好地总结以上催化反应实现肿瘤特异性治疗的研究,推动这一研究方向的进展,该综述在国际上首次提出“纳米催化医学的新概念,并对其本质和应用作了定义和讨论。通过全面总结该研究团队在纳米催化医学领域取得的重要进展,进一步汇集了国内外相关最新研究成果和动向,提出了作者对该领域发展现状的观点,展望了纳米催化医学领域的潜在发展方向。这一工作有望为个性化生物医学提供一种全新且低毒有效的癌症诊疗解决方案,从而为纳米医学领域的发展提供新的研究思路,将对化学、材料、生物和医学领域的交叉研究产生重要的指导意义。
该研究团队已在“纳米催化医学”这一全新领域取得系列重要进展,相关主要研究成果已发表于:Nat. Nanotechnol., 2017, 12, 378-386; Nat. Commun., 2017, 8, 357; J. Am. Chem. Soc., 2017, 139, 1275-1284; J. Am. Chem. Soc., 2016, 138, 9881-9894; J. Am. Chem. Soc., 2014, 136, 16326-16334; Angew. Chem. Int. Ed., 2016, 128, 2141-2146; Adv. Mater., 2015, 27, 4155-4161; Adv. Mater., 2014, 26, 7019-7026; Adv. Funct. Mater., 2014, 24, 4386-4396; Biomaterials, 2017, 125, 23-37; Biomaterials, 2012, 33, 7126-7137等权威学术期刊上。
该工作得到了国家自然科学基金重点项目、面上项目、优秀青年基金项目、国家重点研发计划青年科学家专项等项目的资助和支持。 (来源:生物探索)
Nanoparticle-Triggered In-Situ Catalytic Chemical Reactions for Tumour-Specific Therapy
Abstract Tumour chemotherapy employs highly cytotoxic chemodrugs, which kill both cancer and normal cells by cellular apoptosis or necrosis non-selectively. Catalysing/triggering the specific chemical reactions only inside tumour tissues can generate abundant and special chemicals and products locally to initiate a series of unique biological and pathologic effects, which may enable tumour-specific theranostic effects to combat cancer without bringing about significant side effects on normal tissues. Nevertheless, chemical reaction-initiated selective tumour therapy strongly depends on the advances in chemistry, materials science, nanotechnology and biomedicine. This emerging cross-disciplinary research area is substantially different from conventional cancer-theranostic modalities in clinics. In response to the fast developments in cancer theranostics based on intratumoural catalytic chemical reactions, this tutorial review summarizes the very-recent research progress in the design and synthesis of representative nanoplatforms with intriguing nanostructures, compositions, physiochemical properties and biological behaviours for versatile catalytic chemical reaction-enabled cancer treatments, mainly by either endogenous tumour microenvironment (TME) triggering or exogenous physical irradiation. These unique intratumoural chemical reactions can be used in tumour-starving therapy, chemodynamic therapy, gas therapy, alleviation of tumour hypoxia, TME-responsive diagnostic imaging and stimuli-responsive drug release, and even externally triggered versatile therapeutics. In particular, the challenges and future developments of such a novel type of cancer-theranostic modality are discussed in detail to understand the future developments and prospects in this research area as far as possible. It is highly expected that this kind of unique tumour-specific therapeutics by triggering specific in situ catalytic chemical reactions inside tumours would provide a novel but efficient methodology for benefiting personalized biomedicine in combating cancer.
原文链接:http://pubs.rsc.org/en/content/articlepdf/2018/cs/c7cs00471k




