超强“吃”塑料酶能加速降解饮料瓶
来源:PNAS
作者:John E. McGeehan等
时间:2018-04-25
据英国《独立报》16日报道,英国科学家基于一种酶(生物催化剂),造出了一种能“吃”塑料的物质。新物质有助塑料的回收和再利用,帮助解决全球目前面临的塑料污染问题。
这种酶由生活在日本回收中心的细菌产生。2016年,日本研究人员发现了这种食用塑料的细菌。当时,专家和评论人士就表示,这是解决塑料污染的潜在方法。
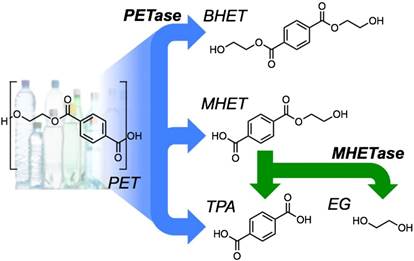
在最新研究中,朴茨茅斯大学生物学家约翰·麦吉汉教授带领团队,对这种酶的结构中与消化塑料有关的部分做了一些微调,造出了这种酶的“超强”版本,其“消化”塑料的能力远超自然界中发现的物质。研究人员将其取名为“PETase”,因为它能分解用于制造饮料瓶的PET塑料,加速这些塑料的降解过程(通常需要数百年时间)。他们表示,通过将塑料分解成易处理的块状物,新物质可以帮助回收数百万吨塑料瓶。
伦敦帝国理工学院化学工程师尼雷·萨哈教授没有参与这项工作,他说:“这种酶对于塑料的回收和再利用非常有用。”
尽管这项发现受到科学家的热烈欢迎,但研究人员也指出,在这些酶广泛应用于回收行业之前,还有很长的路要走。曼彻斯特大学生物分析科学家道格拉斯·凯尔教授说:“石油衍生的塑料和聚合物能抵抗降解,它们在环境中的积累是一个令人困扰的问题,让降解这类塑料的酶不断进化是重中之重。”
最新研究发表在美国《国家科学院学报》上。(来源:科技日报 刘霞)
Characterization and engineering of a plastic-degrading aromatic polyesterase
Abstract Poly(ethylene terephthalate) (PET) is one of the most abundantly produced synthetic polymers and is accumulating in the environment at a staggering rate as discarded packaging and textiles. The properties that make PET so useful also endow it with an alarming resistance to biodegradation, likely lasting centuries in the environment. Our collective reliance on PET and other plastics means that this buildup will continue unless solutions are found. Recently, a newly discovered bacterium, Ideonella sakaiensis 201-F6, was shown to exhibit the rare ability to grow on PET as a major carbon and energy source. Central to its PET biodegradation capability is a secreted PETase (PET-digesting enzyme). Here, we present a 0.92 Å resolution X-ray crystal structure of PETase, which reveals features common to both cutinases and lipases. PETase retains the ancestral α/β-hydrolase fold but exhibits a more open active-site cleft than homologous cutinases. By narrowing the binding cleft via mutation of two active-site residues to conserved amino acids in cutinases, we surprisingly observe improved PET degradation, suggesting that PETase is not fully optimized for crystalline PET degradation, despite presumably evolving in a PET-rich environment. Additionally, we show that PETase degrades another semiaromatic polyester, polyethylene-2,5-furandicarboxylate (PEF), which is an emerging, bioderived PET replacement with improved barrier properties. In contrast, PETase does not degrade aliphatic polyesters, suggesting that it is generally an aromatic polyesterase. These findings suggest that additional protein engineering to increase PETase performance is realistic and highlight the need for further developments of structure/activity relationships for biodegradation of synthetic polyesters.
原文链接:http://www.pnas.org/content/early/2018/04/16/1718804115




