两项成果有助抗糖尿病新药研发并降低药物成本
来源:《自然》
作者:王明伟等
时间:2017-07-04
北京时间5月18日凌晨,国际学术期刊《自然》(论文一、论文二)同时在线发表了我国科学家关于胰高血糖素受体和胰高血糖素样肽-1受体的两项最新研究成果。这两项成果为治疗2型糖尿病和肥胖症的新药研发指明了方向,并可能降低用药成本。
2型糖尿病是世界上患病人数上升最快的疾病之一。胰高血糖素受体和胰高血糖素样肽-1受体是体内血糖代谢的关键调节因子。在饥饿状态下,胰高血糖素受体通过与其配体胰高血糖素结合来提高人体血糖水平;而胰高血糖素样肽-1受体主要在摄食后发挥作用,通过与其配体胰高血糖素样肽-1结合,刺激胰岛素分泌,使餐后血糖降低并维持在正常水平。这一领域的研究是抗糖尿病药物设计和研发需要掌管的关键钥匙。
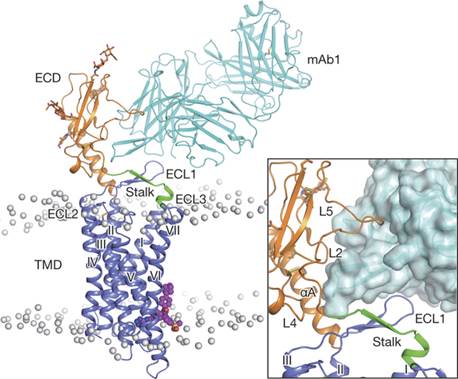
胰高血糖素受体是抗2型糖尿病药物的重要靶点。由于其全长结构信息缺失,影响了靶向该受体的抑制剂药物研发,目前尚无上市药物。这次发布的最新研究成果显示,中科院上海药物研究所领衔的科研团队成功解析人源胰高血糖素受体全长蛋白的三维结构,揭示了该受体蛋白不同结构域对其活化的调控机制,为2型糖尿病治疗新药开发提供了新思路。
胰高血糖素样肽-1受体是公认的2型糖尿病治疗靶标,目前已有多个激动该受体的多肽药物上市,它们的年销售总额超过100亿美元。但当下多肽药物的经典给药方法是注射,靶向该受体口服药物研发一直是国际医药产业关注的热点。
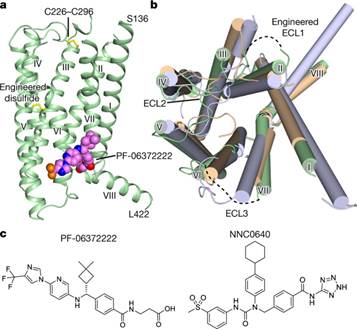
上海科技大学iHuman研究所领衔的科研团队成功解析人源胰高血糖素样肽-1受体七次跨膜区晶体结构,揭示了其别构调节机理,也为相关药物研发奠定了结构生物学基础。
科研团队成员、复旦大学药学院院长王明伟说,希望近3年内在科研成果转化方面实现突破。这些成果成功应用后将降低糖尿病治疗药物的用药成本。(来源:新华社 刘雪 周琳)
Structure of the full-length glucagon class B G-protein-coupled receptor
Abstract The human glucagon receptor, GCGR, belongs to the class B G-protein-coupled receptor family and plays a key role in glucose homeostasis and the pathophysiology of type 2 diabetes. Here we report the 3.0 Å crystal structure of full-length GCGR containing both the extracellular domain and transmembrane domain in an inactive conformation. The two domains are connected by a 12-residue segment termed the stalk, which adopts a β-strand conformation, instead of forming an α-helix as observed in the previously solved structure of the GCGR transmembrane domain. The first extracellular loop exhibits a β-hairpin conformation and interacts with the stalk to form a compact β-sheet structure. Hydrogen–deuterium exchange, disulfide crosslinking and molecular dynamics studies suggest that the stalk and the first extracellular loop have critical roles in modulating peptide ligand binding and receptor activation. These insights into the full-length GCGR structure deepen our understanding of the signalling mechanisms of class B G-protein-coupled receptors.
原文链接:http://www.nature.com/nature/journal/vaop/ncurrent/pdf/nature22363.pdf
Human GLP-1 receptor transmembrane domain structure in complex with allosteric modulators
Abstract The glucagon-like peptide-1 receptor (GLP-1R) and the glucagon receptor (GCGR) are members of the secretin-like class B family of G-protein-coupled receptors (GPCRs) and have opposing physiological roles in insulin release and glucose homeostasis1. The treatment of type 2 diabetes requires positive modulation of GLP-1R to inhibit glucagon secretion and stimulate insulin secretion in a glucose-dependent manner2. Here we report crystal structures of the human GLP-1R transmembrane domain in complex with two different negative allosteric modulators, PF-06372222 and NNC0640, at 2.7 and 3.0 Å resolution, respectively. The structures reveal a common binding pocket for negative allosteric modulators, present in both GLP-1R and GCGR3 and located outside helices V–VII near the intracellular half of the receptor. The receptor is in an inactive conformation with compounds that restrict movement of the intracellular tip of helix VI, a movement that is generally associated with activation mechanisms in class A GPCRs4, 5, 6. Molecular modelling and mutagenesis studies indicate that agonist positive allosteric modulators target the same general region, but in a distinct sub-pocket at the interface between helices V and VI, which may facilitate the formation of an intracellular binding site that enhances G-protein coupling.
原文链接:http://www.nature.com/nature/journal/vaop/ncurrent/pdf/nature22378.pdf




