桃金娘抗耐药菌活性成分研究取得进展
来源:RSC Advances
作者:刘洪新等
时间:2016-09-02
桃金娘科约有130属,4500-5000种,主要分布于地中海地区、马达加斯加、亚洲的热带和温带地区、澳大利亚、太平洋各岛屿以及南美洲热带地区。我国约有10属(包括引入栽培的5属)121种(50个特有种,32栽培种)。桃金娘【Rhodomyrtustomentosa (Ait.) Hassk.】为该科桃金娘属的模式种,也是国内唯一种,是岭南地区分布和用途都非常广泛的药用植物。
中国科学院华南植物园天然产物化学生物学研究组博士刘洪新在研究员邱声祥和助理研究员谭海波的指导下,对桃金娘进行了系统的化学成分研究,发现了一系列新颖结构的间苯三酚类衍生物,主要为间苯三酚与单萜及倍半萜的杂二聚产物,并通过多重波谱解析、ECD计算、X-ray单晶衍射以及全合成等方法确定了这类化合物的化学结构和绝对构型。并且发现tomentosenol A和tomentosone C等化合物有良好的抗革兰氏阳性耐药细活性。
该研究工作陆续发表于化学期刊RSC Advances(2016, 6, 25882-25886; 2016, 6, 48231-48236)和Organic & Biomolecular Chemistry(DOI:10.1039/C6OB01215A)以及Journal of Asian Natural Products Research(2016, 6, 535-541)上。该研究成果得到国家重大科技专项(No.2014ZX10005002-005)、国家自然科学青年基金(No. 81502949)、广东省科技计划(No.2016A010105015)、广东省自然科学基金和博士启动基金(No. 2015A030310482;2016A030313149)的资助。(来源:生物通)
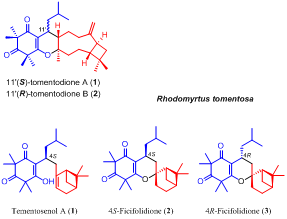
Isolation, synthesis, and biological activity of tomentosenol A from the leaves of Rhodomyrtus tomentosa
Abstract Tomentosenol A (1), along with a pair of epimers, 4S-focifolidione (2) and 4R-focifolidione (3), were isolated from the leaves of Rhodomyrtus tomentosa. 1 was the first example of a new meroterpenoid class with a unique skeleton that contained a free syncarpic acid coupled with a terpenoid unit, and showed excellent antimicrobial and cytotoxic activities. The absolute configuration of 1 was unambiguously determined by a chemical conversion between 1 and the predetermined 2. In contrast to the common hetero Diels–Alder cycloaddition embodied in previously reported meroterpenoid biosynthesis, an Alder-ene reaction was proposed as the key transformation to account for the biosynthesis of 1, which was confirmed by a biomimetic total synthesis.
原文链接:http://pubs.rsc.org/en/content/articlepdf/2016/ra/c6ra01594h
Isolation and biomimetic total synthesis of tomentodiones A–B, terpenoid-conjugated phloroglucinols from the leaves of Rhodomyrtus tomentosa
Abstract Tomentodiones A (1) and B (2), a pair of C-11′ epimers of caryophyllene-conjugated phloroglucinols with an unprecedented skeleton, were isolated from the leaves of Rhodomyrtus tomentosa. Their structures were elucidated through the application of extensive spectroscopic measurements with the absolute configuration of 1 determined by single-crystal X-ray diffraction analysis and electronic circular dichroism (ECD) calculations. The biogenetic pathways of 1 and 2 were proposed to involve an intermolecular, inverse electron demand Diels–Alder cycloaddition reaction as the key step, and their biomimetic total synthesis was accomplished.
原文链接:http://pubs.rsc.org/en/content/articlepdf/2016/ra/c6ra08776k
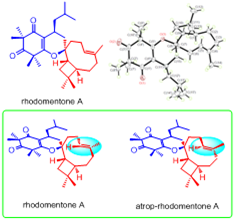
Rhodomentones A and B, novel meroterpenoids with unique NMR characteristics fromRhodomyrtus tomentosa
Abstract Two novel meroterpenoids, rhodomentones A and B bearing an unprecedented caryophyllene-conjugated oxa-spiro[5.8] tetradecadiene skeleton, were isolated from the leaves of Rhodomyrtus tomentosa. Their structures with unique NMR characteristics were determined by extensive spectroscopic analysis, single-crystal X-ray diffraction, quantum molecular calculation, chemical transformation as well as total synthesis.
原文链接:http://pubs.rsc.org/en/content/articlepdf/2016/ob/c6ob01215a
Antimicrobial acylphloroglucinols from the leaves of Rhodomyrtus tomentosa
Abstract Phytochemical study on the leaves of Rhodomyrtus tomentosa resulted in the isolation of fourteen compounds including a new acylphloroglucinol, named tomentosone C (1), and a new flavonol glycoside, namely myricetin-3,7,3′-trimethyl ether-5′-O-β-glucopyranoside (2). Their structures were characterized by spectral data interpretation for new structures and in comparison with published data for known compounds. The antimicrobial activity evaluation revealed that 1 and the known acylphloroglucinol rhodomyrtone (3) exhibited significant antimicrobial activity with MIC 3.66 and 1.83 μg ml−1, respectively, toward Staphylococcus aureus, responsible for the antimicrobial activity observed with the n-hexane and EtOAc-soluble fraction of the ethanol extract of R. tomentosa leaves.
原文链接:http://www.tandfonline.com/doi/full/10.1080/10286020.2015.1121997




