血红素换个“心”,催化大不同
来源:《Nature》
作者:Hanna M. Key等
时间:2016-07-14
酶是以高效率和高选择性而著称的天然生物催化剂,它是大自然最精巧的创造之一。不过纯天然的酶原本是服务于生命体系的,催化反应的种类有限。人工合成的催化剂当然应用范围更广,不过酶的优越特性又实在不忍舍弃,这种时候喜欢不信邪的化学家就要大显身手啦。这不,加州大学伯克利分校(UC Berkeley)的John F. Hartwig教授瞄上了血红蛋白。
血红蛋白的一大特征是拥有Fe-卟啉IX(Fe-PIX)辅因子,原始的血红蛋白可以催化C-H键氧化和卤化反应,并且可以成功地应用于非生物底物。而以Fe-PIX为核心的其他酶,可以催化卡宾、氮宾与烯烃或X-H键的插入反应(X = N, S)和端芳香烯烃的环丙烷化,但对不活泼的C-H键和烯烃的类似反应则无效。
在以往的研究中,科学家已经发现对于一些反应,小分子Ru/Rh/Ir-卟啉的催化效果远好于Fe-卟啉分子,于是他们在想,用这些贵金属代替这些酶中的铁能否收到奇效?
这个想法并非首创,实际上Mn/Cr/Co核心的“血红蛋白”已经被报道过,只是它们的催化活性都不如Fe-PIX,对Ru/Rh/Ir核心的催化效果,John F. hartwig团队的成员心里也没底。(Abiological catalysis by artificial haem proteins containing noble metals in place of iron. Nature, 2016, DOI: 10.1038/nature17968)
计划的第一步是要合成出相应金属核心的酶。已有的方法包括组合法和单一表达法。组合法需要先用酸把血红蛋白的结构打破,释放出Fe-PIX后,再使空的血红蛋白(apo-PIX)折叠回原样。之后再与不同的M-PIX(M为各种金属)混合,得到产物后分离纯化。这一过程非常繁琐,产率低下。另一种单一表达法比较简单,直接表达出M-PIX核心的蛋白,但几乎每一种金属都要换一个表达方法,缺乏普适性,效率依旧不高。于是本文的研究人员开发了一种新的表达系统。
他们尽量降低大肠杆菌培养基中铁盐的含量,以抑制血红素的合成,并保持较低的温度使apo-PIX蛋白保持稳定。最终他们得到了含铁量低于5%的apo-PIX蛋白。这些蛋白与不同的M-PIX混合,填补蛋白中的空位,便能得到不同的M-PIX蛋白。
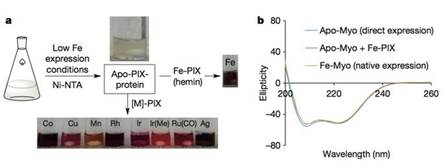
M-PIX蛋白制备过程。圆二色光谱表明细菌中表达的apo-PIX蛋白、加铁后的蛋白与天然的Fe-PIX蛋白在构象上没有差别。
获得了这些M-PIX蛋白后,研究人员比较了不同金属核心的酶催化卡宾的C-H键插入反应及芳香烯烃加成反应的活性。结果表明Ir(Me)-PIX蛋白的催化活性最好。
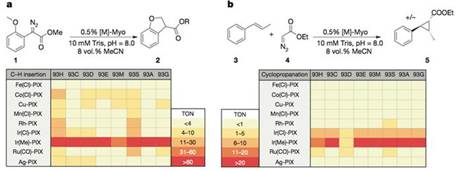
基于此,为了获得高的对映选择性和产率,研究人员又对PIX结合位点附近的重要氨基酸逐一做了突变、筛选(93G代表蛋白质93号氨基酸为甘氨酸G)。
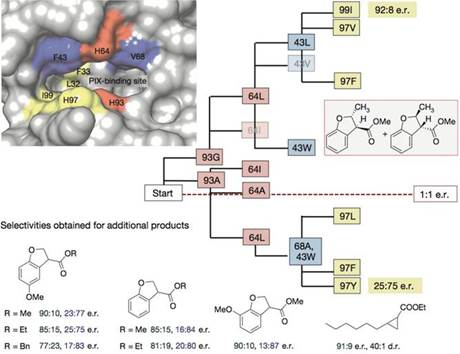
Ir(Me)-PIX蛋白的不同突变体不仅弥补了Fe-PIX蛋白的不足,能够成功催化卡宾的C-H键插入反应和不活泼烯烃加成反应,而且对映选择性、催化剂转换数TON(单位时间内转化的底物的物质的量/催化剂的物质的量)都相当不错。
氘评:
这项简短有力的工作虽然还不够完美,在底物耐受性、产率、选择性、催化效率、机理研究等方面还有很大进步空间,但无疑打开了人工改造天然酶催化剂的新思路。仅仅是把自然界存在的酶换一个金属核心,就能对新的化学反应产生催化功能,不知道是大自然故意给人类留下了发挥空间,还是化学家足够机智呢?(来源:生物360)
Abiological catalysis by artificial haem proteins containing noble metals in place of iron
Abstract Enzymes that contain metal ions—that is, metalloenzymes—possess the reactivity of a transition metal centre and the potential of molecular evolution to modulate the reactivity and substrate-selectivity of the system. By exploiting substrate promiscuity and protein engineering, the scope of reactions catalysed by native metalloenzymes has been expanded recently to include abiological transformations. However, this strategy is limited by the inherent reactivity of metal centres in native metalloenzymes. To overcome this limitation, artificial metalloproteins have been created by incorporating complete, noble-metal complexes within proteins lacking native metal sites. The interactions of the substrate with the protein in these systems are, however, distinct from those with the native protein because the metal complex occupies the substrate binding site. At the intersection of these approaches lies a third strategy, in which the native metal of a metalloenzyme is replaced with an abiological metal with reactivity different from that of the metal in a native protein. This strategy could create artificial enzymes for abiological catalysis within the natural substrate binding site of an enzyme that can be subjected to directed evolution. Here we report the formal replacement of iron in Fe-porphyrin IX (Fe-PIX) proteins with abiological, noble metals to create enzymes that catalyse reactions not catalysed by native Fe-enzymes or other metalloenzymes. In particular, we prepared modified myoglobins containing an Ir(Me) site that catalyse the functionalization of C–H bonds to form C–C bonds by carbene insertion and add carbenes to both β-substituted vinylarenes and unactivated aliphatic α-olefins. We conducted directed evolution of the Ir(Me)-myoglobin and generated mutants that form either enantiomer of the products of C–H insertion and catalyse the enantio- and diastereoselective cyclopropanation of unactivated olefins. The presented method of preparing artificial haem proteins containing abiological metal porphyrins sets the stage for the generation of artificial enzymes from innumerable combinations of PIX-protein scaffolds and unnatural metal cofactors to catalyse a wide range of abiological transformations.
原文链接:http://www.nature.com/nature/journal/vaop/ncurrent/pdf/nature17968.pdf




