绿茶可治疗唐氏综合症
来源:《The Lancet Neurology》
作者:Rafael de la Torre等
时间:2016-06-20

最近一项研究证明,绿茶中的一种化学物质可改善唐氏综合症患者的认知能力。在长达一年的临床试验中,这种治疗方法改善了患者的记忆和行为测试得分,研究人员将相关结果发表在临床神经学顶级杂志《The Lancet Neurology》。在试验结束后六个月,治疗的积极作用仍然存在。脑部扫描显示,这种化合物——称为儿茶素(epigallocatechin gallate),改变了大脑中神经元连接的方式。
本文通讯作者、巴塞罗纳基因组调控中心的研究员Mara Dierssen指出:“这是第一次有研究显示,一种治疗方法可有效改善唐氏综合症患者的认知能力。”
她在一份声明中表示,虽然这项研究非常有意义,但是研究结果不应该被解释成一种“治愈性”的方法。但这也许是一种改善患者生活质量的工具。没有参与这项研究的专家,将这项研究描述为“一项令人兴奋和重要的工作”。与此同时,他们警告说,这些结果必须在额外的试验中进行验证。
唐氏综合征即21-三体综合征,又称先天愚型或Down综合征,是由染色体异常(多了一条21号染色体)而导致的疾病。60%患儿在胎内早期即流产,存活者有明显的智能落后、特殊面容、生长发育障碍和多发畸形。
人类通常有23对染色体,包含了总共25000个蛋白质编码基因。在唐氏综合症中,额外的拷贝可导致21号染色体中的一些基因“过度表达”,从而导致认知能力降低和其他健康问题。
在早期实验中,Dierssen使用模拟唐氏综合症的小鼠,发现抑制其中一个基因DYRK1A,可改善大脑的功能和发育。
“一次飞跃”
但是,基因治疗技术对人类并不可取,所以研究人员转向绿茶提取物。在试验中,研究人员将84名患有唐氏综合症的年轻人分为两组。其中一组服用一种不含咖啡因的绿茶补剂,其含有45%的儿茶素,连同每周进行在线认知训练。另一组患者接受相同的训练,但是摄入外观相似的安慰剂,而不是补剂。这些受试者在3、6和12个月后,进行了认知测试。
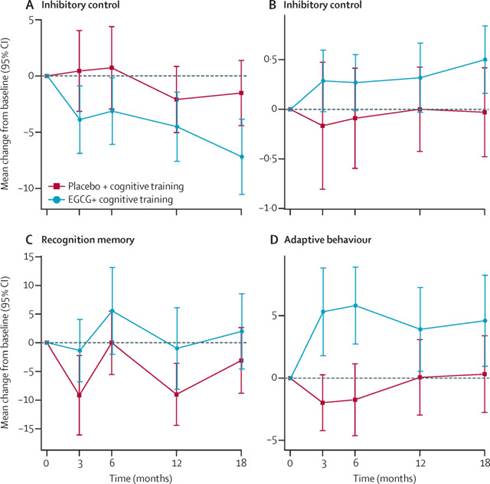
在大部分类别中,很少有甚至没有变化,但是在一些方面——记忆能力模式、口头回忆、适应性行为,“绿茶”受试组的得分显著更好。此外,随着时间的推移他们继续得以改善。
伦敦帝国理工学院神经精神药理学中心的负责人David Nutt这样评论这项研究:“了解唐氏综合症导致的遗传神经生物学,会带来可能的治疗方法,这是令人兴奋的。”
巴黎大脑和脊柱研究所的专家Marie-Claude Potie表示,这些结果是“一次飞跃”,但是其安全性和有效性还有待于确认。
然而,研究人员提醒说,尽管他们承认这项新研究的重要性,但遗传学不是万能的。亚利桑那大学Evelyn F. McKnight大脑研究所的Fabian Fernandez和Jamie Edgin在一篇评论中这样写道:“我们再也不能仅仅通过21三体的镜头来观察唐氏综合症患者。”
他们在《The Lancet Neurology》中写道,同样重要的是,要根据每个人更大的遗传学和环境背景、以及其他健康问题和受教育的机会,来了解他们。
除了用于唐氏综合症治疗之外,绿茶提取物还有其他治疗用途。早在2013年9月,候伯特(Hobart)Menzies研究中心华裔博士生利用老鼠实验发现饮用绿茶的可预防糖尿病。相关阅读:华裔博士生发现绿茶或可预防糖尿病。
2014年10月,发表在《自然纳米技术》杂志上的一项研究发现,绿茶中的一种主要成分能够作为抗癌蛋白载体,用来合成一种稳定、有效的治疗用纳米复合物。相关阅读:从绿茶中开发出抗癌药物;绿茶主要成分或可用作抗癌蛋白载体。(来源:生物通:王英)
Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Down's syndrome (TESDAD): a double-blind, randomised, placebo-controlled, phase 2 trial
Abstract Background Early cognitive intervention is the only routine therapeutic approach used for amelioration of intellectual deficits in individuals with Down's syndrome, but its effects are limited. We hypothesised that administration of a green tea extract containing epigallocatechin-3-gallate (EGCG) would improve the effects of non-pharmacological cognitive rehabilitation in young adults with Down's syndrome.
Methods We enrolled adults (aged 16–34 years) with Down's syndrome from outpatient settings in Catalonia, Spain, with any of the Down's syndrome genetic variations (trisomy 21, partial trisomy, mosaic, or translocation) in a double-blind, placebo-controlled, phase 2, single centre trial (TESDAD). Participants were randomly assigned at the IMIM-Hospital del Mar Medical Research Institute to receive EGCG (9 mg/kg per day) or placebo and cognitive training for 12 months. We followed up participants for 6 months after treatment discontinuation. We randomly assigned participants using random-number tables and balanced allocation by sex and intellectual quotient. Participants, families, and researchers assessing the participants were masked to treatment allocation. The primary endpoint was cognitive improvement assessed by neuropsychologists with a battery of cognitive tests for episodic memory, executive function, and functional measurements. Analysis was on an intention-to-treat basis. This trial is registered with ClinicalTrials.gov, number NCT01699711.
Findings The study was done between June 5, 2012, and June 6, 2014. 84 of 87 participants with Down's syndrome were included in the intention-to-treat analysis at 12 months (43 in the EGCG and cognitive training group and 41 in the placebo and cognitive training group). Differences between the groups were not significant on 13 of 15 tests in the TESDAD battery and eight of nine adaptive skills in the Adaptive Behavior Assessment System II (ABAS-II). At 12 months, participants treated with EGCG and cognitive training had significantly higher scores in visual recognition memory (Pattern Recognition Memory test immediate recall, adjusted mean difference: 6·23 percentage points [95% CI 0·31 to 12·14], p=0·039; d 0·4 [0·05 to 0·84]), inhibitory control (Cats and Dogs total score, adjusted mean difference: 0·48 [0·02 to 0·93], p=0·041; d 0·28 [0·19 to 0·74]; Cats and Dogs total response time, adjusted mean difference: −4·58 s [–8·54 to −0·62], p=0·024;d −0·27 [–0·72 to −0·20]), and adaptive behaviour (ABAS-II functional academics score, adjusted mean difference: 5·49 [2·13 to 8·86], p=0·002; d 0·39 [–0·06 to 0·84]). No differences were noted in adverse effects between the two treatment groups.
原文链接:http://ac.els-cdn.com/S1474442216300345/1-s2.0-S1474442216300345-main.pdf?_tid=3942e1da-3041-11e6-88e2-00000aacb360&acdnat=1465696917_24de2ff3cb55cbd402a384fb619e2cc5




