蛋白质结构新见解或改变生物医学未来
来源:PNAS
作者:Elizabeth Meiering等
时间:2016-01-11
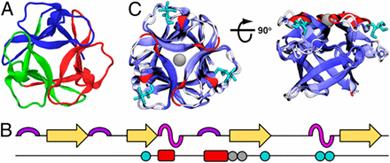
据最新一期《美国国家科学院院刊》报道,加拿大研究人员发现了一种创建设计蛋白质的新方法,或给生物技术和个性化医疗带来全新变革。
滑铁卢大学生物工程和技术中心教授伊丽莎白·梅尔英联合印度、美国研究人员,创建出一个可承受一系列生理及环境条件的蛋白质。而此前,生理及环境条件对科学家寻求创造超稳定、高功能蛋白质造成极大的挑战。
蛋白类药物可被设计成扮演抗体的角色及搜寻特定的细胞。这种个性化药物只在需要的地方发挥作用,从而大大减少了癌症、关节炎等疾病治疗中的副作用。然而,设计出能够承受各种条件的蛋白质既具挑战性又有风险性。蛋白质依靠其独特的结构以执行其功能,结构上的一个小变化即可导致过敏反应,甚至是致命的级联免疫应答。
梅尔英表示,部分正确的蛋白质设计并不足够,必须做到完全正确才能让蛋白质稳定、功能齐全,使药物发挥作用。大多数天然蛋白质都不是很稳定,科学家们发现很难设计蛋白质的稳定性。新成果则使研究人员对蛋白质的设计和理解有了真正的转变。
传统的蛋白质设计要么侧重于结构,要么侧重于功能,梅尔英则通过使用生物信息学,充分挖掘大自然中的信息,使设计过程做到结构和功能并重,然后对可折叠功能性蛋白的展开和发生故障的时间进行测量和分析。
通过组合利用生物物理学和计算机分析,研究团队发现,蛋白质的动力学稳定性可基于蛋白链循环回到其折叠结构自身的程度而建模。由此得到的稳定性是定量的,因此蛋白质的稳定性就可进行调节,当不再需要时便可将其自然分解。
这种迥异的思维方式将允许研究人员以更为精准控制的稳定性开展蛋白设计,从而化解在生物传感器和个性化治疗应用上的挑战。(来源:科技日报 冯卫东)
Designed protein reveals structural determinants of extreme kinetic stability
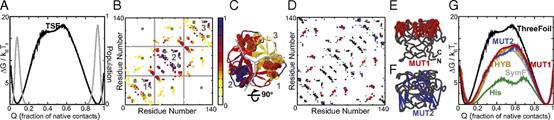
Abstract The design of stable, functional proteins is difficult. Improved design requires a deeper knowledge of the molecular basis for design outcomes and properties. We previously used a bioinformatics and energy function method to design a symmetric superfold protein composed of repeating structural elements with multivalent carbohydrate-binding function, called ThreeFoil. This and similar methods have produced a notably high yield of stable proteins. Using a battery of experimental and computational analyses we show that despite its small size and lack of disulfide bonds, ThreeFoil has remarkably high kinetic stability and its folding is specifically chaperoned by carbohydrate binding. It is also extremely stable against thermal and chemical denaturation and proteolytic degradation. We demonstrate that the kinetic stability can be predicted and modeled using absolute contact order (ACO) and long-range order (LRO), as well as coarse-grained simulations; the stability arises from a topology that includes many long-range contacts which create a large and highly cooperative energy barrier for unfolding and folding. Extensive data from proteomic screens and other experiments reveal that a high ACO/LRO is a general feature of proteins with strong resistances to denaturation and degradation. These results provide tractable approaches for predicting resistance and designing proteins with sufficient topological complexity and long-range interactions to accommodate destabilizing functional features as well as withstand chemical and proteolytic challenge.
原文链接:http://www.pnas.org/content/112/47/14605.full.pdf




