科学家解析青蒿素类过氧桥键合成机制
来源:Nature
作者:Wupeng Yan等
时间:2015-11-12
青蒿素因屠呦呦喜获诺奖一下子走进了公众视野,其“功绩”开始为人所知。虽然近40年来青蒿素治愈了无数疟疾患者,但药物来源却成了老大难。中国科学院微生物研究所973项目《合成微生物体系的适配性研究》课题组发布在3日《自然》杂志在线版的成果称,他们从真菌中解析青蒿素类过氧桥键的生物合成机制,为获取青蒿素提供了一种别样的思路。
最初,青蒿素要通过植物提取,但产量有限,远远满足不了市场的需求。科学家转而尝试用化学方式,但仍未成功应用于商业化生产。近年来,他们开始转向生物合成技术,并在转基因酵母中生产出青蒿酸——青蒿素合成的前体。但研究表明,青蒿素的生物活性与过氧桥键密不可分,换句话说,要想让青蒿酸变成青蒿素,就必须要有过氧桥键“穿线搭桥”。但过氧桥键又必须通过相关催化酶才能生成,因此找到过氧桥键合酶成为了学界的期盼。
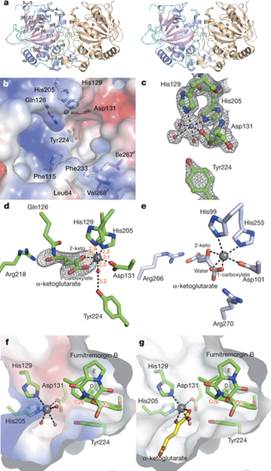
此次,中科院微生物研究所张立新带领的973项目组大胆猜测,过氧桥键合酶可能来源于与黄花蒿共生的真菌中,并试图从自主构建的海洋微生物天然产物库中发现这类含有过氧桥键的化合物及其相应的催化酶。通过与973海外团队成员美国波士顿大学刘平华教授课题组和德克萨斯大学奥斯汀分校张燕教授课题组密切合作,他们从几株曲霉和靑霉中分离出具有抗感染等多种生物活性的含过氧桥键萜类吲哚生物碱化合物震颤真菌毒素,并解析出该化合物中的过氧桥键是由一个依赖a-酮戊二酸的单核非血红素酶FtmOx1催化合成。他们在文章中首次展示了FtmOx1的晶体结构,以及FtmOx1分别与a-酮戊二酸和底物fumitremorgen B的共晶体结构,并通过详尽的酶学实验证实了FtmOx1的功能。
研究人员认为,阐明这一特别的过氧桥键的生物合成新机制,为发现催化青蒿酸形成青蒿素的过氧桥键合酶向前迈进了一大步。(来源:科技日报)
Endoperoxide formation by an α-ketoglutarate-dependent mononuclear non-haem iron enzyme
Abstract Many peroxy-containing secondary metabolites have been isolated and shown to provide beneficial effects to human health. Yet, the mechanisms of most endoperoxide biosyntheses are not well understood. Although endoperoxides have been suggested as key reaction intermediates in several cases, the only well-characterized endoperoxide biosynthetic enzyme is prostaglandin H synthase, a haem-containing enzyme. Fumitremorgin B endoperoxidase (FtmOx1) from Aspergillus fumigatus is the first reported α-ketoglutarate-dependent mononuclear non-haem iron enzyme that can catalyse an endoperoxide formation reaction. To elucidate the mechanistic details for this unique chemical transformation, we report the X-ray crystal structures of FtmOx1 and the binary complexes it forms with either the co-substrate (α-ketoglutarate) or the substrate (fumitremorgin B). Uniquely, after α-ketoglutarate has bound to the mononuclear iron centre in a bidentate fashion, the remaining open site for oxygen binding and activation is shielded from the substrate or the solvent by a tyrosine residue (Y224). Upon replacing Y224 with alanine or phenylalanine, the FtmOx1 catalysis diverts from endoperoxide formation to the more commonly observed hydroxylation. Subsequent characterizations by a combination of stopped-flow optical absorption spectroscopy and freeze-quench electron paramagnetic resonance spectroscopy support the presence of transient radical species in FtmOx1 catalysis. Our results help to unravel the novel mechanism for this endoperoxide formation reaction.
原文链接:http://www.nature.com/nature/journal/vaop/ncurrent/pdf/nature15519.pdf




