一个信号通路有望减缓大麻癌症治疗中的副作用
来源:《PLos Biology》
作者:Xavier Vi?als等
时间:2015-07-28

近期,东安格利亚大学与位于巴塞罗那的庞培法布拉大学的科学家们合作发现了一种减缓大麻医疗过程中副作用的方法。研究已经在老鼠身上验证,并寄希望应用于肿瘤医疗,从而避免因大麻治疗造成的情绪、认知和记忆的负影响。相关研究成果发表于《PLOS BIOLOGY》。
大麻可以作为一种药物应用于肿瘤治疗。研究发现,大麻内含有一种成分:四氢大麻酚(THC),能够有效抑制肿瘤生长。东安格利亚大学药学院的彼得•麦考密克博士说:“THC作为大麻的主要活性成分,具有广泛的医疗用途,包括缓解疼痛、恶心和焦虑。我们先前的研究还发现,它可以减少癌症患者的肿瘤大小,但是也会产生不少副作用,例如记忆力损伤、忧虑和依赖性严重。
有大量医学研究致力于探索THC调控的分子机制,以便发挥它的医学功效的同时消除或缓解其副作用。麦考密克和他的研究团队发现THC介导一个独立的信号途径。该通路包含两个受体:大麻素受体和5-羟色胺受体。当任一受体途径受阻后,THC仍然通过另一受体途径可以发挥效用,例如缓解疼痛。最新的研究在此基础上探究如何避免药物的副作用。
研究小组通过对小鼠试验研究大脑在THC调控下如何反应,结果表明,当特定的5-羟色胺受体(5HT2AR)没有时,能减缓THC的副作用,例如记忆损伤。但是当降低5HT2AR含量,不能减缓其他包括疼痛在内的副作用。
综上所述,这项研究发现一个信号通路,能够有望解决目前医学治疗面临的THC存在副作用的难题,具有重大的意义。同时,麦考密克博士也强调,患者不应该使用大麻给自己治疗。他希望他们的研究能够指引未来医疗朝着安全的方向发展。(来源:生物探索)
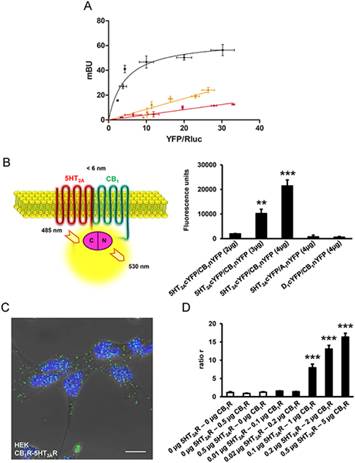
Abstract Activation of cannabinoid CB1 receptors (CB1R) by delta9-tetrahydrocannabinol (THC) produces a variety of negative effects with major consequences in cannabis users that constitute important drawbacks for the use of cannabinoids as therapeutic agents. For this reason, there is a tremendous medical interest in harnessing the beneficial effects of THC. Behavioral studies carried out in mice lacking 5-HT2A receptors (5-HT2AR) revealed a remarkable 5-HT2AR-dependent dissociation in the beneficial antinociceptive effects of THC and its detrimental amnesic properties. We found that specific effects of THC such as memory deficits, anxiolytic-like effects, and social interaction are under the control of 5-HT2AR, but its acute hypolocomotor, hypothermic, anxiogenic, and antinociceptive effects are not. In biochemical studies, we show that CB1R and 5-HT2AR form heteromers that are expressed and functionally active in specific brain regions involved in memory impairment. Remarkably, our functional data shows that costimulation of both receptors by agonists reduces cell signaling, antagonist binding to one receptor blocks signaling of the interacting receptor, and heteromer formation leads to a switch in G-protein coupling for 5-HT2AR from Gq to Gi proteins. Synthetic peptides with the sequence of transmembrane helices 5 and 6 of CB1R, fused to a cell-penetrating peptide, were able to disrupt receptor heteromerization in vivo, leading to a selective abrogation of memory impairments caused by exposure to THC. These data reveal a novel molecular mechanism for the functional interaction between CB1R and 5-HT2AR mediating cognitive impairment. CB1R-5-HT2AR heteromers are thus good targets to dissociate the cognitive deficits induced by THC from its beneficial antinociceptive properties.
原文链接:http://www.plosbiology.org/article/fetchObject.action?uri=info:doi/10.1371/journal.pbio.1002194&representation=PDF




