植物“入睡”依靠两种酶作为“开关”
来源:PNAS
作者:Keisuke Yoshida等
时间:2018-08-23
许多植物在白天进行光合作用,到了晚上光线较弱的时候也会“入睡”。日本一项新研究说,植物“入睡”要依靠两种酶作为“开关”,这个发现有望用于设计能适应不同环境的作物。
植物通过叶绿体进行光合作用。过去的研究发现,植物在白天光线变强时会“醒来”,叶绿体增强活动,而在晚上光线变暗后又会“入睡”,叶绿体减弱活动,这样可以使光合作用的总体效率较高,避免浪费能量。此前研究人员曾发现了让植物“醒来”的机制,但对植物“入睡”的机制并不清楚。
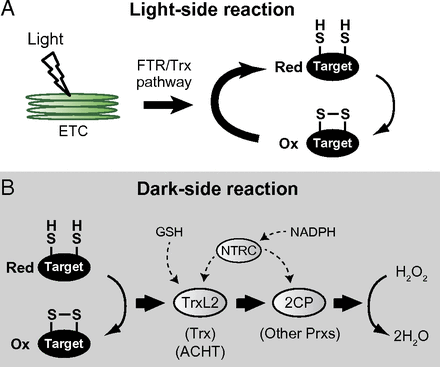
日本东京工业大学研究人员在新一期美国《国家科学院学报》上发表报告说,两种分别名为TrxL2和2CP的酶共同发挥了让植物“入睡”的“开关”作用。植物体内一些蛋白质在“醒来”时会被还原,这两种酶能让它们重新被氧化,从而让植物“入睡”。氧化还原反应的循环也使得植物在“睡”与“醒”间循环。
用拟南芥进行的实验显示,如果抑制这两种酶的作用,则拟南芥无法在晚上“入睡”,叶绿体会在光线较暗的情况下进行效率不高的光合作用。
研究人员说,这项发现阐明了植物在夜间防止能量浪费的机制,给植物科学基础研究带来新突破,有望为今后设计环境适应型作物提供方法。(来源:新华社 华义)
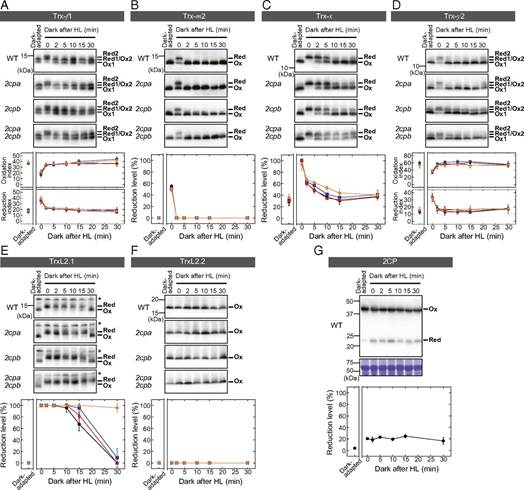
Thioredoxin-like2/2-Cys peroxiredoxin redox cascade supports oxidative thiol modulation in chloroplasts
Abstract Thiol-based redox regulation is central to adjusting chloroplast functions under varying light conditions. A redox cascade via the ferredoxin-thioredoxin reductase (FTR)/thioredoxin (Trx) pathway has been well recognized to mediate the light-responsive reductive control of target proteins; however, the molecular basis for reoxidizing its targets in the dark remains unidentified. Here, we report a mechanism of oxidative thiol modulation in chloroplasts. We biochemically characterized a chloroplast stroma-localized atypical Trx from Arabidopsis, designated as Trx-like2 (TrxL2). TrxL2 had redox-active properties with an unusually less negative redox potential. By an affinity chromatography-based method, TrxL2 was shown to interact with a range of chloroplast redox-regulated proteins. The direct discrimination of thiol status indicated that TrxL2 can efficiently oxidize, but not reduce, these proteins. A notable exception was found in 2-Cys peroxiredoxin (2CP); TrxL2 was able to reduce 2CP with high efficiency. We achieved a complete in vitro reconstitution of the TrxL2/2CP redox cascade for oxidizing redox-regulated proteins and draining reducing power to hydrogen peroxide (H2O2). We further addressed the physiological relevance of this system by analyzing protein-oxidation dynamics. In Arabidopsis plants, a decreased level of 2CP led to the impairment of the reoxidation of redox-regulated proteins during light–dark transitions. A delayed response of protein reoxidation was concomitant with the prolonged accumulation of reducing power in TrxL2. These results suggest an in vivo function of the TrxL2/2CP redox cascade for driving oxidative thiol modulation in chloroplasts.
原文链接:http://www.pnas.org/content/early/2018/08/07/1808284115




