令抗癌化疗药物降低副作用的新技术
来源:《Cancer Research》
作者:王杭祥等
时间:2018-01-16
抗癌化疗药物毒副作用太大,病人不堪重负,是否有新的技术和方法减轻药物副作用?今日来自来自浙江大学医学院附属第一医院郑树森院士王杭祥课题组的研究人员发表了题为“New Generation Nanomedicines Constructed from Self-Assembling Small-Molecule Prodrugs Alleviate Cancer Drug Toxicity”的论文,阐述了运用小分子前药技术结合纳米自组装构建的超分子纳米药物,极大地降低化疗小分子药物的毒副作用,为药物的临床转化提供了新的思路。
这一研究成果公布在Cancer Research杂志,郑树森院士为论文的通讯作者,第一作者兼并列通讯作者为王杭祥副研究员。
紫杉烷类药物是临床广泛使用的一类化疗药物,其中卡巴他赛更是因其能够克服紫杉醇及多西紫杉醇的耐药性以及高效杀伤肿瘤细胞的能力,在2010年被美国FDA批准用于临床。然而其在临床上表现出较大毒性,临床I期研究表明该药物的最大耐受剂量(MTD)仅为25 mg/m2,远低于其同族药物紫杉醇(175 mg/m2)及多西紫杉醇(60-100 mg/m2)的最大耐受剂量。此外,卡巴他塞水溶性差,临床处方中含有的增溶剂吐温-80具有溶血性和其他副作用。
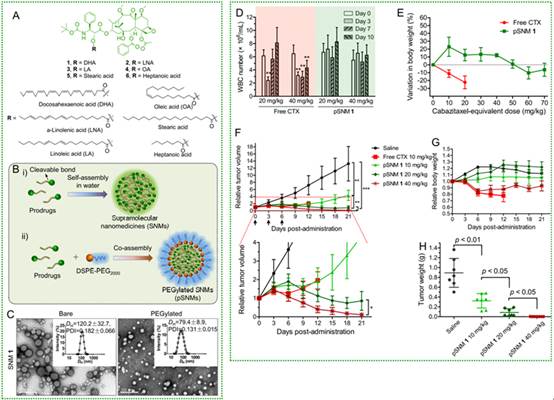
为此,在最新研究中,研究人员采用药物分子重构及自组装技术,改善卡巴他赛的临床使用价值。课题组针对卡巴他赛分子2’位羟基的活泼性,选用多种脂肪酸对其进行了酯化,获得若干小分子前药(图A)。
令人神奇的是,经不饱和脂肪酸修饰后的小分子前药具备在水溶液中自组装形成超分子纳米结构的能力,该超分子纳米颗粒溶于水可用于临床静脉滴注(图B和C)。经进一步研究表明,卡巴他赛超分子纳米药物不仅在动物体内表现出极小的系统性毒性和骨髓抑制能力(MTD可提高7倍以上,图D和E),而且在移植瘤动物模型中超分子药物保留了卡巴他赛原药的抑瘤能力,没有降低药效(图F、G和H)。
通过该技术制备得到的超分子药物与传统的小分子药物相比具有如下明显优势:首先,能够通过自组装形成具有水溶性的药物纳米颗粒,解决了卡巴他赛难溶性的问题,避免了药物在临床使用时需要吐温-80及乙醇等助溶剂带来的毒性问题。
其次,小分子药物自组装后形成的纳米粒载药量高,可达69%。再次,自组装药物在体内水解释放出卡巴他赛及不饱和脂肪酸,而不饱和脂肪酸又是为人体所必需,对人体无害甚益之。然后,通过大量的动物实验,课题组发现卡巴他赛的体内副作用被极大地降低。最后,小分子前药化学结构明确,由小分子自组装构建的纳米药物制备简易,满足临床大批量生产的要求,因此具备较好的临床转化前景。(来源:生物通)
New Generation Nanomedicines Constructed from Self-Assembling Small-Molecule Prodrugs Alleviate Cancer Drug Toxicity
Abstract The therapeutic index for chemotherapeutic drugs is determined in part by systemic toxicity, so strategies for dose intensification to improve efficacy must also address tolerability. In addressing this issue, we have investigated a novel combinatorial strategy of reconstructing a drug molecule and using sequential drug-induced nanoassembly to fabricate supramolecular nanomedicines (SNM). Using cabazitaxel (CTX) as a target agent, we established that individual synthetic prodrugs tethered with polyunsaturated fatty acids were capable of recapitulating self-assembly behavior independent of exogenous excipients. The resulting SNM could be further refined by PEGylation with amphiphilic copolymers suitable for preclinical studies. Among these CTX derivatives, docosahexaenoic acid-derived compound 1 retained high anti-proliferative activity. SNM assembled with compound 1 displayed an unexpected enhancement of tolerability in animals along with effective therapeutic efficacy in a mouse xenograft model of human cancer, compared to free drug administered in its clinical formulation. Overall, our studies showed how attaching flexible lipid chains to a hydrophobic and highly toxic anticancer drug can convert it to a systemic self-deliverable nanotherapy, preserving its pharmacological efficacy while improving its safety profile.
原文链接:http://cancerres.aacrjournals.org/content/canres/early/2017/10/20/0008-5472.CAN-17-0984.full.pdf




